The teeth of the extant tiger shark Galeocerdo cuvier are among my favorite shark teeth for two primary reasons: their overall shape (shared by others, all extinct, in this genus) and their serrations, wonderfully abundant in this particular species. This post has gone through myriad permutations, beginning with an attempt to capture my confusion over the scientific literature on this species' serrations (difficult to write and uninteresting to read). I have decided, instead to write briefly about what I think I know about the serrations of the G. cuvier which includes a discussion of the descriptive terminology applied to them, as well as a short review of what I understand about their function.
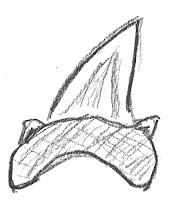
G. cuvier, the sole living member of this species, is a large, dangerous denizen of the world’s oceans. It dates back to the Pliocene Epoch (5.3 to 2.6 million years ago) (some research may push the shark’s origins into the mid-Miocene Epoch, perhaps 13.8 million years ago). (For the earlier date, see Türtscher, 2021. All references are presented at the end of this post.) G. cuvier teeth have a striking morphology, often and appropriately described as cockscomb-shaped. It is quite distinctive and, so, it's hard to confuse these teeth with those from other species. The illustration below captures that recognizable shape. Three key aspects of that shape are marked: the mesial cutting edge, the distal cutting edge, and distal heel. The front of the mouth is at the left in this image. (This image is based on one appearing in Türtscher, 2022.)

That each (extant and extinct) species in the Galeocerdo genus features some array of serrations renders them irresistible. But G. cuvier teeth are very special in that regard: not only is the entire outer edge of their crown serrated, but the serrations on both its mesial cutting edge and distal heel are themselves serrated. Serrated serrations! What’s not to like?
The first picture below shows the lingual (tongue) side of a tooth from a modern adult G. cuvier. The distance from edge to edge of the very bottom of the tooth is 25 mm. The second picture shows the labial (lip) side of an adult G. cuvier from the Yorktown Formation at the Lee Creek Mine. The measurement across the bottom is the same as for the first tooth. This particular specimen dates from the Pliocene Epoch.
Serration Terminology
The scientific terms used to distinguish among the general characteristics of shark dentition are challenging, very specialized, and quite alien. To wit, polphyodonty (teeth are continuously replaced throughout the life of the shark), homodonty (teeth are all the same shape), heterodonty (there are different tooth shapes in the same individual), monognathic heterodonty (tooth shape changes moving from front to back along the jaw), dignathic heterodonty (different shapes in the upper jaw compared to the lower jaw), and gynandric heterodonty (teeth of the same species differ in shape by sex). (Türtscher, 2022.) Some of these characteristics vary in the same species depending upon such factors as age or mating season. Not surprisingly, these are terms that I come to this literature prepared to decipher (a cheat sheet helps).
The G. cuvier dentition is largely the same morphologically from front to rear and top jaw to bottom jaw. They do vary by ontogenetic stage of development, particularly with regard to the extent and robustness of the serrations. (Türtscher, 2022, p. 10.)
Though these scientific terms present a hurdle to the casual reader of this literature, my attention is drawn repeatedly to the various (and sometimes inconsistent) uses of adjectives to describe the serrations on these teeth.
Primary and secondary are the most precisely applied adjectives. Indeed, a key distinction for serrations on G. cuvier teeth is between two types: primary serrations and secondary serrations. The large serrations that occur on the mesial cutting edge and the distal heel of these teeth are primary serrations. The smaller serrations that line the distal cutting edge and also appear on the edges of (and between) the primary serrations on the mesial cutting edge and distal heel are called secondary serrations. These different types of serrations are marked below in the closeup of the distal cutting edge and part of the distal heel of the specimen shown in the first picture above.

In their study of serrated shark teeth (blue shark, white shark, and tiger shark), Moyer and Bemis name and distinguish these two types of G. cuvier serrations. Significantly, their analysis shows an underlying histological difference between them. Primary serrations consist of three layers of enameloid with a partial internal filling of dentine; secondary serration generally have only two of the three enameloid layers and no infilling dentine. (Moyer and Bemis, 2017, p. 105.) I will outline later what Moyer and Bemis posit regarding the function of these secondary serrations.
Once I move beyond that central distinction in the kinds of serrations on the G. cuvier adult teeth, there’s no consistency in the terms used but in most cases context suggests intended meaning.
Serrations with serrations. These have been described as compound (Kent, 2018, p. 108), complex (Kent, 1994, p. 96; Türtscher, 2022, p.12), and double (Cappetta, 1987, p. 17; Türtscher, 2022, p. 2).
Serrations without serrations. These have been called simple (Kent, 1994, p. 96) and singly serrated (Türtscher, 2021, p. 584).
A bit more problematic is that these various adjectives are mixed and matched in different ways. For instance, Cappetta distinguishes between simple and double serrations. (Cappetta, 187, p. 17.) Then there’s the application of the adjective compound to describe the overall configuration of all serrations on the G. cuvier, which are a combination of different types of serrations. Türtscher and her colleagues do this in the following description of adult G. cuvier teeth:
The crown is completely serrated with compound serrations, whereby large primary serrations are located on the mesial cutting edge and the distal heel, while secondary serrations are situated on and between primary serrations as well as on the distal cutting edge. (Türtscher, 2022, p. 3.)
Function of G. cuvier Serrations
Why serrations and why serrated serrations?
The teeth of G. cuvier are characterized as cutting type teeth, a category of tooth that includes broad and relatively flat teeth with a cusp that often is curved toward the rear of the mouth. Moyer and Bemis argue that stress on the G. cuvier tooth concentrates on the large notch where the distal cutting edge meets the distal heel (see outline of the G. cuvier tooth above). This, they suggest, may help the tooth cut through tough prey. (Moyer and Bemis, 2017, p. 107.) That’s quite relevant given that the adult diet does focus on tough prey, such as mammals, other sharks, rays, and sea turtles. (Türtscher, 2022, p. 12.)
Adding serrations to these teeth boosts their effectiveness. (Cappetta, 1987, p. 16-17.) Frazzetta concludes: “Serrated teeth can make greater use of the available biting forces, and they have greater cutting effect than do smooth-edged teeth.” (Frazzetta, 1988, p. 93.)
But serrated serrations apparently are a rarity, so, to what end did they appear in the Galeocerdo lineage? The secondary serrations on the edges of the primary serrations may make the cutting edges more efficient when the tiger shark engages in its typical vigorous head shaking when attacking prey. The notches marking the juncture of secondary and primary serrations may also serve as points at which stress is concentrated, reducing the wear on the primary serrations, helping to keep them functional longer. (Moyer and Bemis, 2017, p. 107-108.) Ultimately, for the G. cuvier, secondary serrations “are probably linked to the prominence of hard-shelled prey in its diet, specifically sea turtles, whose shells are composites of bone and keratin, presenting a unique challenge to predators.” (Moyer and Bemis, 2017, p. 109; see, also, Türtscher, 2022, p. 12.)
I think the importance of secondary serrations, including those that run along the edges of the primary serrations on the G. cuvier’s mesial cutting edge and distal heel, requires a bit of elaboration. The Galeocerdo, like all sharks, is polyphyodont, that is, it is constantly replacing its teeth. As a result, the protective role that secondary serrations may play for primary serrations need last only until the tooth is replaced. That’s actually quite efficient considering its prey.
Closing with reference to the G. cuvier’s diet is appropriate, I think. Yes, its teeth are wonderfully structured for the role they play in enabling this fish to consume its prey, but this shark is not picky. In addition to its living prey, it adds carrion and a very wide variety of trash to what it swallows, thereby living up to its sobriquet: “A garbage can with fins.” (Compagno, 2005, p. 308.)
References
Cappetta, H., Handbook of Paleoichthyology: Chondrichthyes II: Mesozoic and Cenozoic Elasmobranchii, 1987.
Compagno, Leonard, et al., Sharks of the World, Princeton Field Guide, 2005.
Frazzetta, T.H., The Mechanics of Cutting and the Form of Shark Teeth (Chondrichthyes, Elasmobranchii), Zoomorphology, Volume 108, 1988.
Kent, Bretton W. Fossil Sharks of the Chesapeake Bay Region, 1994.
Kent, Bretton W. The Cartilaginous Fishes (Chimaeras, Sharks, and Rays) of Calvert Cliffs, Maryland, USA, chapter 2 in The Geology and Vertebrate Paleontology of Calvert Cliffs, Maryland, USA, edited by Stephen J. Godfrey, Smithsonian Contributions to Paleobiology, Number 100, 2018.
Moyer, Joshua K., and Bemis, William E., Shark Teeth as Edged Weapons: Serrated Teeth of Three Species of Selachians, Zoology, Volume 120, 2017.
Türtscher, Julia, et al., Evolution, Diversity and Disparity of the Tiger Shark Lineage Galeocerdo in Deep Time, Paleobiology, Volume 47, Number 4, 2021.
Türtscher, Julia, et al., Heterodonty and Ontogenetic Shift Dynamics in the Dentition of the Tiger Shark Galeocerdo cuvier (Chondrichthyes, Galeocerdidae), Journal of Anatomy, April, 2022.